Effect of low-intensity broadband ultrasound on breast cancer cells line in vitro
Kuzmenko A.P.1, Marchenko A.T.2
Summary. Study of the effect of different modes of low-intensity ultrasound on the cisplatin-resistant breast cancer cell line MCF-7/DDP in vitro. Materials and methods. Studies were conducted on MCF-7/DDP breast cancer cells. The source of ultrasonic vibrations was the SDG 2082 X Generator of the company Siglent. 2 modes of ultrasound exposure were used, which differed in intensity and breast cancer cells in 4-well plates were sonicated for 5 minutes over 5 days, followed by counting of live and dead cells in the Goryaev chamber and assessment of genotoxicity using cytological tests (CT). Cytological studies were performed using an Olympus BX-41 microscope (Olympus Europe GmbH, Japan). Cells were stained with trypan blue and acridine orange. The results. Features of the cytotoxic effect of various types of low-intensity ultrasound on the cisplatin-resistant human breast cancer cell line MCF-7/DDP are shown. Low-intensity pulsed ultrasound (LIPUS) increased the cytotoxicity index by 4 times, and low-intensity ultra-broadband ultrasound (UMUS) by 8 times compared to the control (p <0.05). The effect on the culture of UMUS micro cells (the intensity of the effect was reduced by several orders of magnitude) was not accompanied by cytotoxic effects. The obtained data are confirmed by CT-indicators. Conclusions. The application of LIPUS and UMUS ultrasonic exposure was accompanied by a significant increase in cytotoxicity and increased mutagenic changes in the nuclei of MCF-7/DDP cells. The cytotoxic effect is most pronounced when using UMUS on MCF-7/DDP cancer cells.
Received 29.04.2024
Accepted for publication 15.05.2024
DOI: 10.32471/clinicaloncology.2663-466X.54-2.32382
INTRODUCTION
Mechanobiology, which has emerged in recent years, considers the exchange of mechanical signals in biological tissues and cells as one of the main mechanisms for controlling biological functions [1]. According to Shannon’s formula, the maximum amount of information or energy E that can be transferred from object to object is:
E = Δf • log2 (1 + Wsignal/Wnoise),
where Δf is a frequency band, Wsignal and Wnoise — signal and noise power.
The formula shows that for better control of biological functions it is better to increase the bandwidth than the signal energy, since the signal energy is under the sign of the logarithm [2]. Recently, a new Ultra-wideband Micro-energy Ultrasound technology has emerged [3]. The search for possible ways of influencing new therapeutic signals on tumor processes in oncology is relevant. Tumor cell culture can serve as a convenient and relatively simple model of the tumor process, which makes it possible to quickly study the influence of various factors on the viability and metabolism of tumor cells. Recently, work has appeared on the effect of LIPUS on tumor cells, showing the possibility of inhibiting their proliferation [4–7]. The purpose of this research was a comparative study of the effect of UMUS and LIPUS on MSF-7/DDR breast cancer cells resistant to cisplatin.
MATERIALS AND METHODS
The research material was the cell line MCF-7/DDP (from human breast cancer cells, resistant to cisplatin). The cells were obtained from the human and animal cell lines and tissue bank of the R.E. Kavetsky Institute of Experimental pathology, Oncology and Radiobiology of the NAS of Ukraine. The cells were cultured in DMEM nutrient medium with 10% fetal calf serum, 40 μg/ml gentamicin in a humidified atmosphere with 5% CO2 at 37 °C. After the cells formed a dense monolayer on the substrate (3 days of growth), they were repassaged. To do this, cells were separated from the substrate using a phosphate-buffered saline PBS solution with 1 mM EDTA. The following types of influence were used in the studies: signal parameters are shown in Table 1. To excite the ultrasonic transducer an SDG 2082 X Generator from Siglent Company was used as a source of electrical signals. Ultra-wideband conversion of generator signals into ultrasonic vibrations in the frequency range 1–7 MHz was carried out by a 15-mode piezoceramic transducer with overlapping vibration modes and polarization normal to the radiating surface. The transducer’s diameter is 20 mm with a maximum thickness of 3 mm. Copper electrodes to the working and back sides of the transducer were applied. The front surface was protected by a wear-resistant multilayer coating made of layers of titanium, stainless steel and titanium nitride. The impact of LIPUS and UMUS was carried out directly on the bottom of a 4-well plate through a layer of ultrasonic gel Aqua Ultra Basic UBQ 5000 Ultragel (Hungary). The cell suspension was planted on 4-well plates (well diameter 20 mm) at a concentration of 1•104 cells/well in 1 ml of complete growth medium. After 2, 24, 48, 72 and 96 hours cells were treated with the parameters given in Table 1 and were incubated under standard conditions for 96 hours. The exposure to ultrasonic waves was 5 minutes.
| Groups | 2 | 3 | 4 |
| Ultrasound type | LIPUS | UMUS micro | UMUS |
| −10 dB Bandwidth | 10 kHz | 6 MHz | 6 MHz |
| Frequencies, MHz | 1.5 | 1–7 | 1–7 |
| Spectrum feature | – | All frequency
simultaneously |
All frequency
simultaneously |
Intensity, mW/cm2:
|
30 | 0.01 | 30 |
| Pulse duration, s | 2•10-4 (200 µs) | 5•10-8 (50 ns) | 5•10-8 (50 ns) |
| Pulse repetition rate, kHz | 1 | 10 | 10 |
| Duration of the procedure, min | 5 | 5 | 5 |
| Number of procedures | 5 | 5 | 5 |
After incubation of the cells, 100 μl of the versen preparation was added to each well of a 4-well plate and incubated for 5 minutes at 37 °C after 96 hours the cells were counted in a Goryaev chamber. Cells were stained with trypan blue ex tempore and the number of living and dead cells in the field of view of a Karl Zeiss optical microscope was counted.
To assess the mutagenic effect of the ultrasonic vibrations used, the generally accepted СT was used [8]. After incubating the cells 100 μl of versene was added to each well of a 4-well plate and incubated for 5 minutes at 37 °C, after which the cells were counted in a Goryaev chamber. A smear was made on glass slides and fixed with methanol. Cells were stained with acridine orange ex tempore and the number of vesicles was analyzed under a fluorescence microscope. Microscopy (light and luminescence) was carried out using an Axiostar Plus microscope from Karl Zeiss (Germany).
Statistical analysis was carried out using Student’s t-test with preliminary testing of the hypothesis about the normal distribution of a random variable using the Kolmogorov — Smirnov test using the STATISTICA 6.0 software package (StatSoft).
RESULTS
As a result of the studies, it was established that sonication of the MCF-7/DDP cell line in the UMUS micro group did not lead to a significant increase in the number of dead cells compared to the control (Table 2). When using the LIPUS and UMUS the number of dead cells was significantly higher (p <0.05) compared not only with the control, but also with the UMUS micro. This may be due to the fact that the intensity of ultrasound radiation in the UMUS micro was three orders of magnitude lower (0.01 mW/cm2 and 30 mW/cm2) than in the LIPUS. It is important to note that there was a significant increase in the number of dead cells when exposed to UMUS compared to all other study groups (p <0.05). The results obtained may indicate a significant cytotoxic effect of the LIPUS and UMUS on MCF-7/DDP cells, especially pronounced when using UMUS. At the same time, the power of ultrasonic radiation in these was the same and amounted to 30 mW/cm2. There was no increase in the number of living cells when using these modes, which may indicate a predominance of cytotoxic factors over proliferative ones. It is also interesting that according to the literature, the use of the LIPUS led to an increase in the proliferation of unchanged cells (fibroblasts), accelerated the time of epithelization of wound surfaces and reparative processes of bone tissue [9–11]. This multidirectional effects when using ultrasound in the LIPUS mode are due to different mechanisms when affecting normal cells and cells with malignant transformation.
| Ultrasound type | Quantitysamples | Quantityliving cells (average) | Quantitydead cells (average) | % of dead cells(average) |
| Control | 6 | 94±4.34 | 3.8±0.97 | 3.73±0.75 |
| UMUS micro | 6 | 86.2±5.89 | 5.2±0.86 | 5.67±0.95 |
| LIPUS | 6 | 90.8±8.61 | 17.4±3.37 | 16.4±2.98*, ** |
| UMUS | 6 | 62±4.79 | 31±7.31 | 31.5±5.42*, ** |
*p <0.05 compared to control. **p <0.05 compared to UMUS micro.
When analyzing the results using the CT on MCF-7/DDP cells, it was found that sonication in the UMUS micro mode was accompanied by the lowest values (Table 3) which may indicate the absence of a mutagenic effect on the cell genome. In other study groups (LIPUS and UMUS) an increase in CT values was observed, especially in the UMUS group (p <0.05). The data obtained correlate with indicators of cellular cytotoxicity (Table 3). We can conclude that the cytotoxicity we observed in the LIPUS and UMUS groups can be realized due to the mutagenic effect of the ultrasound types under study.
| Ultrasound type | Number samples | Mutagenicity indicator MT per 1000 cells (average) | Morphological description |
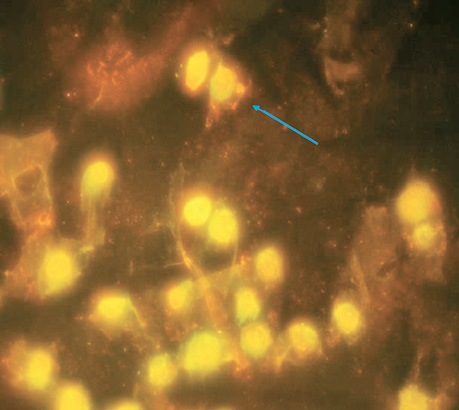
| Control | 5 | 4±0.16 | The norm for this cell culture (Fig. 1). |
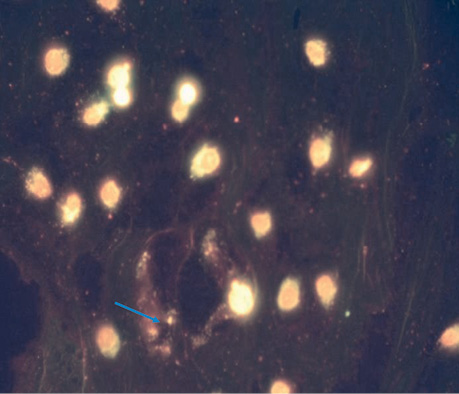
| UMUS micro | 5 | 2.8±0.37 | The nuclei are clearly defined and do not differ from the control. Single cases of blebbing, many cells in the early stages of division (Fig. 2). |
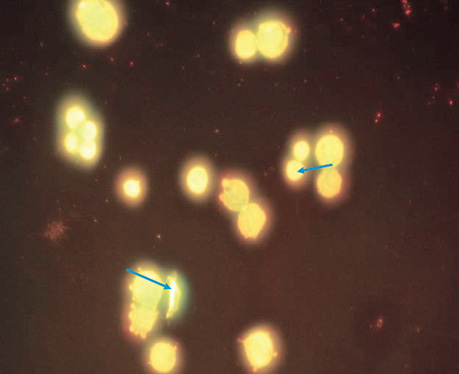
| LIPUS | 5 | 5.2±0.58 | Blebbing was detected in 40% of cells. In approximately 20% of cells, the nucleus does not have a clear shape, fragmentation of the genetic material is observed, some cells have 2 or more vesicles, but despite this. The core size is not larger than normal (Fig. 3). |
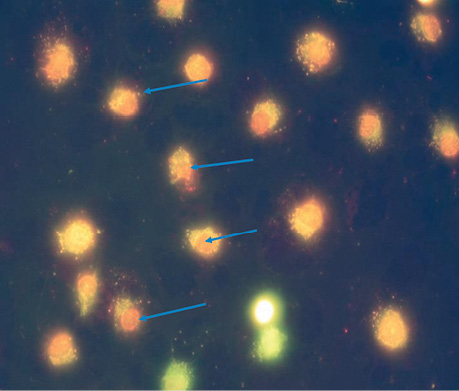
| UMUS | 5 | 9.5±0.86*, ** | The nuclei of most cells (>80%) do not have a clear shape; the genetic material is diffusely scattered around clear vesicles; apoptotic changes are observed in almost all cells; the number of vesicles with a high % of dead cells indicates their death with genetic abnormalities (Fig. 4). |
*p <0.05 compared to LIPUS and UMUS.
**p <0.05 compared to LIPUS, UMUS micro and control.
CONCLUSIONS
Sonication of the MCF-7/DDP cell line with ultra-wideband ultrasonic micro-intensity (UMUS micro) did not lead to a cytotoxic and mutagenic effect.
The use of LIPUS and UMUS types accompanied by a significant increase in the cytotoxic effect and an increase in mutagenic changes in the nuclei of MCF-7/DDP cells. Stimulation of proliferative processes when exposed to LIPUS on normal cells is known, however, in our studies, the use of LIPUS was accompanied by a cytotoxic effect when exposed to transformed cells of the MCF-7/DDP line. The cytotoxic effect is most pronounced when UMUS is applied to cancer cells of the MCF-7/DDP line.
Conflict of interest.
The authors declare no conflict of interest.
The authors express their gratitude to the doctor of sciences N.O. Bezdenezhniy and PhD O.O. Likhova R.E. Kavetsky Institute of Experimental pathology, Oncology and Radiobiology of the NAS of Ukraine for help in conducting research.
REFERENCEs
1. Argentati, C., Morena, F., Bazzucchi, M., Armentano, I., Emiliani, C., & Martino, S. (2019). Insight in to Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions. International Journal of Molecular Sciences, 20, 5337. doi: 10.3390/ijms20215337.
2. Shennon, C. E. (1947). A Mathematical Theory of Communication. Bell System Technical Journal, 27, 379–423.
3. Marchenko, A., & Mictukevictus, A. (2024). Device for Ultra-wideband Micromechanical Therapy and Method of its Operations (U.S. Patent № 2024/0009487A1).
4. Wood, A. K., & Sehgal, C. M. (2015). A review of low-intensity ultrasound for cancer therapy. Ultrasound in Medicine and Biology, 41, 905–928. doi: 10.1016/j.ultrasmedbio.2014.11.019.
5. Matsuo, T., Sato, K., Matsui, T., Sawada, S., Muramatsu, Y., Kawanami, K., & Deie, M. (2017). Inhibitory effects of low-intensity pulsed ultrasound sonication on the proliferation of osteosarcoma cells. Oncology Letters, 14(3), 3071–3076. doi: 10.3892/ol.2017.6472.
6. Xia, Y., Yang, M., Xiao, X., Tang, W., Deng, J., Wu, L., … Wang, Y. (2024). Low-intensity pulsed ultrasound activated the anti-tumor immunity by irradiating the spleen of mice in 4T-1 brest cancer. Cancer Immunology, 73(3), 50. doi: 10.1007/s00262-023-03613-1.
7. Carina, V., Costa, V., Pagani, S., De Luca, A., Raimondi, L., Bellavia, D., … Giavaresi, G. (2018). Inhibitory effects of low intensity pulsed ultrasound on osteoclastogenesis induced in vitro by breast cancer cells. Journal of Experimental & Clinical Cancer Research, 37(1), 197. doi: 10.1186/s13046-018-0868-2.
8. Fenech, M., Knasmueller, S., Bolognesi, C., Bonassi, S., Holland, N., Migliore, L., … Kirsch-Volders, M. (2016). Molecular mechanisms by which in vivo exposure to exogenous chemical genotoxic agents can lead to micronucleus formation in lymphocytes in vivo and ex vivo in humans. Mutation Research/Reviews in Mutation Research, 770(Pt A), 12–25. doi: 10.1016/j.mrrev.2016.04.008.
9. de Oliveira, P. D., Pires Oliveira, D. A., Martignago, C., & Poli-Frederico, R. C. (2015). Effect of low-intensity pulsed ultrasound therapy on a fibroblast cell culture. Fisioterapia e Pesquisa, 22(2), 112–118.
10. Harrison, A., Lin, S., Pounder, N. & Mikuni-Takagaki, Y. (2016). Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics, 70, 45–52. doi: 10.1016/j.ultras.2016.03.016.
11. Marchenko, A., & Kuzmenko, A. (2024). The influence of low-intensity broadband ultrasound on skin regeneration in experiment. Grail of Science, 38, 142–146. doi: 10.36074/grail-of-science.12.04.2024.022.
Correspondence:
Alexander Kuzmenko
9 Pyrohova str., Kyiv, 02000
Dragomanov Ukrainian State University
E-mail: kuzmen_alex@ukr.net
Адреса для листування:
Кузьменко Олександр Петрович
02000, Київ, вул. Пирогова, 9
Український державний університет ім. М.П. Драгоманова
E-mail: kuzmen_alex@ukr.net














Leave a comment