Analysis of the relationship between Ki-67 expression dynamic and RECIST 1.1 dynamic during neoadjuvant breast cancer chemotherapy. Prediction of morphological response
- 1Municipal Non-Commercial Enterprise «City Clinical Hospital № 4» of Dnipro City Council, Ukraine
- 2Dnipro State Medical University, Ukraine
Summary. Introduction. Neoadjuvant chemotherapy (NACT) plays a crucial role in the multidisciplinary approach to treating breast cancer (BC), aimed at controlling tumor growth and achieving a complete morphological response. However, there is a concern about the risk of selecting more aggressive tumor cell clones due to intensive treatment. This concern makes identifying prognostic markers before NAC critical for assessing recurrence-free survival. Ki-67, a protein marker of cellular proliferation, is a significant indicator of tumor aggressiveness and chemotherapy effectiveness. High expression of Ki-67 may indicate not only a more aggressive tumor but also a higher likelihood of an effective response to chemotherapy. Information on Ki-67 expression and other clinical and molecular factors can significantly help tailor treatment strategies. Monitoring changes in Ki-67 levels before and after chemotherapy can provide essential insights into the tumor’s response to treatment. Materials and methods. The study included 114 patients who underwent preoperative chemotherapy, and the goal of the study is to determine the correlation between radiological response and morphological response to treatment by formulating a statistical hypothesis. Conclusions. The study confirms a correlation between the dynamics of RECIST 1.1 status and changes in Ki-67 expression, indicating their interdependence as processes that reflect the efficacy of the therapeutic impact on the tumor. Particularly significant is the connection in patients with luminal-B BC, highlighting the importance of analyzing radiological parameters as predictors of treatment response and reduction in the tumor’s proliferative ability. These results could have significant clinical relevance, emphasizing the need to integrate data on tumor size and molecular markers, such as Ki-67, in evaluating the response to NACT. This approach allows for a more accurate prediction of treatment outcomes and adapting therapeutic strategies to patients’ needs. The research supports using a comprehensive approach to assess the prognosis of treatment response in BC patients, integrating clinical, radiological, and molecular parameters to ensure the best treatment strategies and increase the chances of therapy success.
Received 05.03.2024
Accepted for publication 20.03.2024
DOI: 10.32471/clinicaloncology.2663-466X.53-1.31896
Introduction
NACT is an essential component of the comprehensive treatment strategy for BC and can significantly influence the overall strategy and outcomes. It is a systemic treatment method before tumor removal, with the primary goal of reducing tumor volume or achieving complete pathology response (pCR), which allows for the reduction of the surgery volume and improves the prognosis of overall and recurrence-free survival. Identifying prognostic markers before the start of neoadjuvant treatment is an essential issue for predicting tumor response. Between 3 to 10% of patients do not respond to neoadjuvant therapy and progress during preoperative chemotherapy. The primary resistance of the tumor mainly explains this. In such cases, a reasoned approach is the re-biopsy and re-determination of the tumor’s receptor status. In practice, this often occurs during surgical intervention. As a rule, clinical or radiological progression does not affect the change in surgical tactics [1].
Biomarkers from specific protein complexes can be used to predict the tumor’s response to therapeutic interventions. One such protein marker is Ki-67. It is often used as a measure of cellular proliferation or the rate of cell division. In the context of BC, this indicator is usually considered to determine the tumor’s aggressiveness and make decisions regarding treatment tactics [2]. Its expression is a prognostic factor for chemotherapy efficacy [3].
High levels of Ki-67 are generally associated with a higher risk of recurrence and lower overall survival. However, a favorable response to chemotherapy (reducing the marker) can improve the prognosis. According to the international working group, Ki-67 has clinical validity, but its clinical utility is established only for prognosis evaluation. In the study of estrogen-receptor-positive, Her-2/neu-negative tumors, three groups were distinguished: 1) low expression level (≤5%); 2) intermediate (5–30%); and 3) high (>30%). It was found that high levels of Ki-67 were associated with lower recurrence-free survival, both in patients with affected lymph nodes (hazard ratio (HR) 1.59; 95% confidence interval (CI) 1.35–1.87) and in diseases with unaffected lymph nodes (HR 2.31; 95% CI 1.83–2.92), as well as worse overall survival rates in BC patients with positive nodes (HR for death 2.33; 95% CI 1.83–2.95) and with negative nodes (HR 2.54; 95% CI 1.65–3.91) [4, 5].
There is a proportional relationship between the rate of cell division in the tumor, the higher Ki-67 index, and the effectiveness of chemotherapy. The change in Ki-67 expression after NACT for BC can vary in each specific case and depends on many factors, including the individual biological characteristics of the tumor, the type of BC (immunohistochemical subtype), the chemotherapy regimens used, the age of the patient, and comorbidities. Three scenarios of treatment response development should be considered:
Decrease in Ki-67: This is often considered a positive response to treatment, as it indicates that chemotherapy effectively reduces the proliferation rate of tumor cells. A significant decrease in Ki-67 indicates a chemotherapeutic effect and may increase the chances of a more radical outcome from surgical intervention.
Stable Ki-67: In some cases, the marker expression may remain relatively stable after NACT. This suggests that chemotherapy does not significantly impact the cell proliferation rate. However, other factors, such as changes in tumor size or other molecular markers, may still indicate a positive response to treatment.
Increase in Ki-67: Although less common, there are cases where Ki-67 expression may increase after therapy. This could indicate either a lack of control over tumor growth by the chemotherapy, requiring consideration of alternative treatment strategies, or heterogeneity within the initially defined tumor and greater aggressiveness of the residual tumor.
It is important to note that while changes in Ki-67 expression can provide valuable information, they are only part of the puzzle in assessing NACT efficacy for BC. Other factors, such as clinical and radiological evaluations, therapeutic damage to the tumor, and conversion of receptor status, collectively also play a crucial role in determining the effectiveness of treatment [6].
Ultimately, the interpretation of changes in Ki-67 expression should be carried out by a multidisciplinary team (tumor board or MDT) that includes clinical oncologists, chemotherapists, surgeons, and pathologists who consider all available clinical, radiological, and pathological data to make informed decisions regarding the current treatment plan for each patient. It should be noted that each listed factor is prognostically important and can be decisive in making the overall treatment strategy and adjusting the current tactic. A clear example is the conversion of the tumor’s receptor status. Quite often, a change or conversion of hormonal receptors and the receptor for human epidermal growth factor 2 (HER2) is observed when comparing primary tumors with metastases in the breast. Receptor conversion during the oncogenesis of BC is one of the critical factors affecting patient survival. Significant is the fact that patients who have undergone receptor conversion could significantly benefit from adjusting the treatment strategy. This underscores the necessity of precise monitoring of the tumor’s molecular profile at all stages of the disease (initial diagnosis and post-operative biopsy material) to optimize therapeutic approaches [7–9].
In the context of neoadjuvant hormonal therapy for BC, the re-determination of Ki-67 to assess dynamics can play a decisive role and indicate the need to change the tactics of pharmacological treatment. It should be noted that any positive tumor response, such as a reduction in its size, a decrease in Ki-67 expression, therapeutic damage to the tumor, etc., increases the chances of recurrence-free and overall survival of patients [10].
Using ultrasound diagnostics (US) and/or mammography is widely accepted for primary visualization and assessment of dynamics in local stages of BC [11].
The role of computed tomography (CT) in the initial disease assessment and disease control evaluation remains undetermined. According to selective data, CT and/or positron emission tomography (PET-CT) is not routinely performed to diagnose primary BC; however, in some subtypes, it may correlate with histopathological variants of the primary tumor [12].
Indications for performing a CT scan include localized bone pain or elevated blood alkaline phosphatase levels, liver function impairment, abdominal or pelvic pain, symptoms of respiratory system involvement (cough, hemoptysis, dyspnea, etc.), and stage III–IV cancer according to American Joint Committee on Cancer (AJCC) criteria. However, in the latter case, if PET-CT is available and covered by national programs and/or private insurance, this method of investigation is preferred [13, 14].
The common practice involves the initial marking of lesions followed by their evaluation according to RECIST 1.1 criteria in the context of NACT for BC. However, the choice of imaging method should be the one that proved to be the most informative during initial diagnosis and preoperative treatment. Additionally, the process of assessing dynamics may depend on the financial capability of a specific medical center or the healthcare system as a whole.
A crucial issue is the lack of correspondence between tumor imaging data and the final morphological burden. This discrepancy can be related to different models of tumor response to neoadjuvant treatment: symmetric reduction of tumor mass (concentric narrowing), formation of fibrosis, complete resorption of tumor mass, and preservation of microscopic foci of the invasive component. Many patients with a complete radiological response may have residual invasive disease [15].
Some studies confirm the association between radiological and pathomorphological responses of the tumor. Still, this connection should be considered probable because during treatment, the cancer may be replaced by granuloma-like or fibrous tissue. This transformation can affect the accuracy of radiological assessments in predicting the pathomorphological outcome, highlighting the complexity of interpreting imaging results in the context of neoadjuvant treatment for BC [16].
Ultimately, only the surgical stage of treatment, followed by the examination of the tumor bed, can definitively determine the dynamics, results of the morphological response, and conversion of receptor status. This underscores the importance of surgical intervention and subsequent pathological analysis in the comprehensive assessment of treatment efficacy and tumor behavior in response to neoadjuvant therapy for BC [17].
One issue that requires further study is the relationship between the radiological response of the tumor and the reduction of Ki-67. This can be used as an indirect prognostic marker indicating the effectiveness of the preoperative treatment administered. The most pressing question is the dependency between the radiological and morphological responses in those BC subtypes with a statistically lower likelihood of response to NACT, such as luminal-B BC. According to a meta-analysis, the frequency of achieving a pCR based on all 52 studies was 21.1% (range: 10.1–74.2%), with the highest pCR rates observed in HER2+ tumors — 36.4% (range: 17.5–74.2%) and TN (triple-negative) tumors — 32.6% (range: 20.3–62.2%), and the lowest in HR+/HER2- tumors — 9.3% (range: 5.5–31.3%). This highlights the variability in treatment response across different BC subtypes and underscores the need for personalized treatment strategies based on tumor biology and predictive markers [18, 19].
For HER2-positive/hormone-negative BC treated without the use of trastuzumab, the frequency of pCR is 30.2%, whereas for hormone-positive/HER2-negative BC, the rate was 16.2%. This distinction highlights the impact of targeted therapies and the biological characteristics of the tumor on the effectiveness of NACT, demonstrating the significance of HER2 status in predicting treatment response and the necessity for tailored therapeutic approaches based on tumor subtype [20].
Within the scope of a scientific project by the Department of Oncology and Medical Radiology at Dnipro State Medical University, research is being conducted on the efficacy of adding metformin to standard NACT regimens for BC. One of the study’s objectives is to identify prognostic markers of treatment efficacy in both the control and experimental groups. This research explores the potential benefits of integrating metformin, a commonly used medication for type II diabetes, with existing BC treatment protocols, potentially offering a novel approach to enhancing therapeutic outcomes through the modulation of metabolic pathways [21].
Aim
To investigate the relationship between the morphological and radiological responses of the tumor by formulating a statistical hypothesis and conducting statistical analysis to determine the correlation.
Materials and Methods
A null and alternative hypotheses have been formulated to achieve the set objective.
The null hypothesis (H0) asserts that there is no correlation between the dynamics of RECIST 1.1 status upon completion of treatment and the dynamics of the immunohistochemical marker Ki-67 for predicting the tumor’s response to NACT.
The alternative hypothesis (H1) states that there is a correlation between the dynamics of RECIST 1.1 status upon completion of treatment and the dynamics of the immunohistochemical marker Ki-67 for predicting the tumor’s response to NACT.
The study included 114 BC patients treated at the Municipal Clinical Hospital № 4 of Dnipro Municipal Council from November 2020 to January 2024. The patients were in stages I–III at the beginning of the treatment (T1-4, N1-3, M0, G1-3, according to the AJCC 8th edition of 2018). They required NACT as decided by the MDT (order of the institution № 31). Manual examination, radiological study (CT with intravenous contrast), and data from histological and immunohistochemical studies were used to establish the stage.
A core needle biopsy was performed under infiltrative analgesia with sonological navigation. An initial assessment of the immunohistochemical subtype and determination of Ki-67 status were conducted for all patients according to the current protocol using «Vitro master diagnostica — Rabbit Anti-Human Ki-67 Monoclonal Antibody (Clone SP6) MAD-000310QD-7», made in Spain.
The initial tumor size was determined according to RECIST 1.1 criteria by the longest axis for breast lesions and the shortest axis for lymph nodes. An interim assessment of dynamics was conducted in the middle of the planned treatment course (after 2–4 cycles of NACT), and the final evaluation of disease dynamics was carried out on the 10–14th day after the last NACT course. Radiological evaluations were performed using RADIANT Dicom Viewer CD/DVD software version 2023.1 [22].
Surgical treatment followed by histological examination of the tumor was carried out no earlier than 21 days and no later than 42 days after the last course of chemotherapy, provided the patient had fully recovered somatically and laboratory-wise. Most often, the surgery was performed during the fourth week after the end of NACT. All patients included in the study received treatment based on taxanes with or without anthracyclines (4AC + 4T or 4AC +12T, 4–8 cycles of TC) with or without the addition of trastuzumab (depending on the overexpression or amplification of HER2). This approach reflects the tailored treatment strategy aimed at optimizing therapeutic outcomes by considering the specific characteristics of the tumor, including its HER2 status, which is crucial for guiding targeted therapies like trastuzumab [23–25].
Statistical analysis was conducted using the software Minitab 19, Product version: Minitab® 19.1.1 (64-bit). For the mathematical calculation of radiological dynamics for each case, the formula «а/b•100−100» was used, where «a» is the final measurement of marker lesions, and «b» is the initial measurement of marker lesions. To determine the dynamics of the Ki-67 marker, the formula «100−(c•100/d)» was utilized, where «c» is the Ki-67 measurement in postoperative material, and «d» is the initial measurement in biopsy material.
Patient consent for treatment diagnostic procedures, including invasive ones, was obtained following Order № 751 of the Ministry of Health of Ukraine, dated September 29, 2012. Considering that the data presented in this work are part of the scientific research of the Department of Oncology and Medical Radiology of Dnipro State Medical University, informed consent was collected from each patient for participation in the study, including conducting surveys via «Google Forms» regarding adverse effects of the treatment. This approach ensures ethical compliance and patient rights protection in the research process.
Results and Discussion
Statistical analysis was conducted on the NACT results of 114 patients. By immunohistochemical subtypes, the study group was distributed as follows: no cases of luminal-A phenotype — 0% (n=0); luminal B-Her-2/neu negative constituted 44.74% (n=51); luminal-B-Her-2/neu positive constituted 18.42% (n=21); Her-2/neu positive constituted 10.52% (n=12); triple-negative constituted 26.32% (n=30). The average Ki-67 before treatment started was 43.31%, with a mode of 34%. The average Ki-67 after treatment completion was 13.25%, with a mode of 0%. A pCR was achieved by 54 patients, which is 47.36% of the total cases. Among the group of patients with luminal-B BC, pCR was achieved by 27 patients, which is 36.99% of the entire group (according to the literature, achieving a complete morphological response to NACT ranges from 9.3 to 16.2%).
The average sum of marker sizes of primary tumors and marker lymph nodes before treatment, according to initial CT diagnostics, was 56.07 mm. The average sum of marker sizes of primary tumors and marker lymph nodes at the first CT (after 2–4 courses from the start of treatment) control was −32.54 mm, which was −41.7% by RECIST 1.1 criteria. The same indicator after the completion of NACT was 22.85 mm, which by RECIST 1.1 criteria equals −59.48%.
A complete radiological response (corresponding to a complete clinical response) by RECIST 1.1 criteria was achieved by 19 patients, constituting 16.66%, and a partial radiological response was achieved by 76 patients, making up 66.7%, stabilization was achieved by 19 patients, which is 16.66%. Radiological progression, as a sign of primary tumor resistance during treatment, was established in only one case. Still, it was determined from the interim CT control (+24.6%), and when determining from the initial measurement, the total increase was 3.44%. Of the 19 patients who achieved a complete radiological response, 84% also achieved pCR. Among those who achieved a partial radiological response, 42.1% (n=32) achieved pCR.
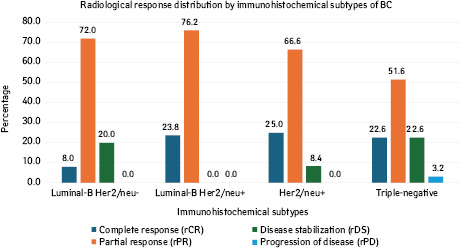
Depending on the immunophenotype of the tumor, the radiological (clinical) response to NACT was distributed as follows (Fig. 1). Thus, in patients with luminal-B Her2/neu negative BC, a complete radiological response (cRR) occurred in 8%, a partial radiological response (pRR) in 72%, and radiological disease stabilization (rDS) in 20%; in women with luminal-B Her2/neu positive BC, cRR occurred in 23.8%, PR in 76.2%, and rDS in 0%; in women with Her2/neu positive BC, cRR occurred in 25%, rPR in 66.6%, and rDS in 8.4%; in women with triple-negative BC, cRR occurred in 22.58%, rPR in 51.61%, rDS in 22.58%, and radiological progression of the disease in 3.23%.
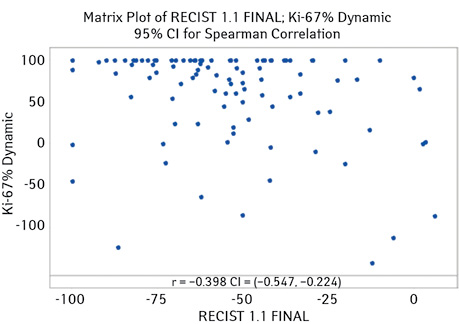
Thus, for all studied immunophenotypes of BC, a correlation was found between the dynamics of Ki67 level and the tumor response according to RECIST 1.1 criteria, which was −0.398 (95% CI for ρ (−0.547; −0.224), p-value <0.001) (Fig. 2).
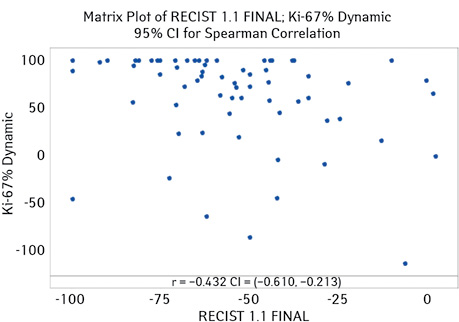
The highest correlation was observed in patients with luminal-B BC (both phenotypes). For this group of patients, the correlation coefficient was −0.432 (95% CI for ρ (−0.610; −0.213), p-value <0.001) (Fig. 3).
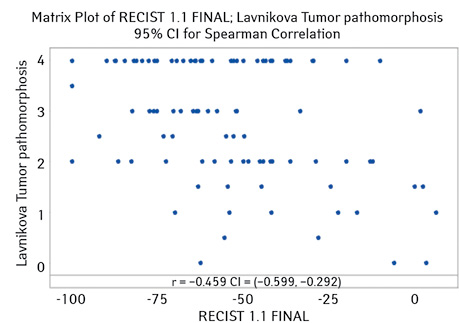
Additionally, the correlation between the degree of therapeutic pathomorphosis of the tumor (according to G.O. Lavnikova) was studied. This indicator was −0.459 (95% CI for ρ (−0.599; −0.292), p-value <0.001) (Fig. 4).
Conclusions
Based on the correlation calculations between the dynamics of tumor sizes according to RECIST 1.1 criteria and the proliferation marker Ki-67 at the beginning and end of treatment for predicting the tumor response to NACT, we can conclude that the null hypothesis (H0) is rejected. The alternative hypothesis (H1) is accepted.
The results of the calculations obtained are statistically significant. The negative correlation coefficient (−0.398) indicates an inverse relationship between the dynamics of RECIST 1.1 status and Ki-67 level and therapeutic pathomorphosis of the tumor: with an improvement in radiological response (decrease in tumor size), there is a decrease in the proliferative activity of the cancer (decrease of level Ki-67) and an increase in therapeutic damage to the cancer.
The correlation is higher for luminal-B BC, at −0.432, and even higher for determining the overall pathomorphosis with radiological response at −0.459. However, since the points are scattered throughout the graph, this may indicate the presence of other variables affecting treatment outcomes that are not accounted for in this analysis.
These results confirm the hypothesis that the reduction in tumor size and the decrease of level Ki-67 are interconnected processes that reflect the effectiveness of the therapeutic impact on the tumor, especially in the group of patients with luminal-B BC, and highlight the importance of this subtype as a significant predictor of treatment response. The high p-values in all three analyses show that the correlations are statistically significant, confirming the conclusions and indicating the possibility of using these parameters as biomarkers for predicting treatment efficacy in patients with BC.
Clinical Implementation
These results have important clinical significance, as they confirm the feasibility of using a combined assessment of the response to neoadjuvant treatment by considering the tumor response according to RECIST 1.1 criteria and the Ki-67 proliferation marker in patients with BC. They allow not only the evaluation of the dynamics of tumor sizes but also the consideration of changes at the molecular level, which can contribute to a more accurate prediction of treatment outcomes and the adaptation of therapeutic strategies.
Information on conflict of interest. The authors declare no conflict of interest.
Protection of Humans and Animals in Research
The work is part of a dissertation and a segment of the planned comprehensive scientific research work of the Department of Oncology and Medical Radiology of the State Institution «Dnipro State Medical University», «Improving personalized methods of systemic treatment taking into account their clinical and molecular-genetic characteristics», state registration number 0117 U 003384, implementation term: 01.2017–11.2022». The meeting of the Committee on Biomedical Ethics was held on 07.12.2020.
The work does not contain unconventional human interventions. There is no risk to research subjects during the performance of the work. Participants involved in the research are informed about all aspects related to the research’s purpose, tasks, methodologies, and expected benefits. Laboratory and instrumental research methods are generally accepted, and the drugs used are approved. Experimental human studies were not conducted. The work complies with all morals and ethics. Norms by ICH/GCP rules, the Helsinki Declaration (1975), the Council of Europe Convention on Human Rights and Biomedicine, and Ukraine’s legislation.
References
1. Myller, S., Ipatti, P., Jääskeläinen, A., Haapasaari, K. M., Jukkola, A., & Karihtala, P. (2020). Early progression of breast cancer during neoadjuvant chemotherapy may predict poorer prognoses. Acta oncologica (Stockholm, Sweden), 59(9), 1036–1042. doi.org/10.1080/0284186X.2020.1760350.
2. Davey, M. G., Hynes, S. O., Kerin, M. J., Miller, N., & Lowery, A. J. (2021). Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers (Basel),13(17), 4455. doi: 10.3390/cancers13174455. PMID: 34503265; PMCID: PMC8430879.
3. Seber, E. S., Iriagac, Y., Cavdar, E., Karaboyun, K., Avci, O., Yolcu, A., … Hacibekiroglu, I. (2024). Efficacy of Neoadjuvant Chemotherapy in Lobular and Rare Subtypes of Breast Cancer. Journal of the College of Physicians and Surgeons-Pakistan: JCPSP, 34(1), 37–41. doi.org/10.29271/jcpsp.2024.01.37.
4. de Azambuja, E., Cardoso, F., de Castro, G. Jr., Colozza, M., Mano, M. S., Durbecq, V., … Paesmans, M. (2007). Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. British journal of cancer, 96(10), 1504–1513. doi.org/10.1038/sj.bjc.6603756.
5. Stuart-Harris, R., Caldas, C., Pinder, S. E., & Pharoah, P. (2008). Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast (Edinburgh, Scotland), 17(4), 323–334. doi.org/10.1016/j.breast.2008.02.002.
6. Zhang, A., Wang, X., Fan, C., & Mao, X. (2021). The Role of Ki67 in Evaluating Neoadjuvant Endocrine Therapy of Hormone Receptor-Positive Breast Cancer. Front Endocrinol (Lausanne), 12, 687244. doi: 10.3389/fendo.2021.687244. PMID: 34803903; PMCID: PMC8597938.
7. Zhao, W., Sun, L., Dong, G., Wang, X., Jia, Y., & Tong, Z. (2021). Receptor conversion impacts outcomes of different molecular subtypes of primary breast cancer. Therapeutic advances in medical oncology, 13, 17588359211012982. doi.org/10.1177/17588359211012982.
8. Yau, C., Osdoit, M., van der Noordaa, M., Shad, S., Wei, J., de Croze, D., … Symmans, W. F. (2022). Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. The Lancet. Oncology, 23(1), 149–160. doi.org/10.1016/S1470-2045(21)00589-1.
9. Elkhazhzh, M. H., Bondarenko, I. M., Zavizion, V. F., Kunyk, A. V., & Aseev, O. I. (2013). The Impact of Systemic Treatment on the Immunophenotype of Breast Cancer. University Clinic, 9(1), 54–58.
10. Smith, I., Robertson, J., Kilburn, L., Wilcox, M., Evans, A., Holcombe, C., … Dowsett, M. (2020). Long-term outcome and prognostic value of Ki-67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol., 21(11),1443–1454. doi: 10.1016/S1470-2045(20)30458-7. Erratum in: Lancet Oncol., 21(12), e553. PMID: 33152284; PMCID: PMC7606901.
11. Kong, X., Zhang, Q., Wu, X., Zou, T., Duan, J., Song, S., … Li, Z. (2022). Advances in Imaging in Evaluating the Efficacy of Neoadjuvant Chemotherapy for Breast Cancer. Front Oncol., 12, 816297. doi: 10.3389/fonc.2022.816297.
12. Paydary, K., Seraj, S. M., Zadeh, M. Z., Emamzadehfard, S., Shamchi, S. P., Gholami, S., … Alavi, A. (2019). The Evolving Role of FDG-PET/CT in the Diagnosis, Staging, and Treatment of Breast Cancer. Mol Imaging Biol., 21(1), 1–10. doi: 10.1007/s11307-018-1181-3. PMID: 29516387.
13. Dayes, I. S., Metser, U., Hodgson, N., Parpia, S., Eisen, A. F., George, R., … Levine, M. N. (2023). Impact of 18F-Labeled Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography Versus Conventional Staging in Patients With Locally Advanced Breast Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 41(23), 3909–3916. doi.org/10.1200/JCO.23.00249.
14. Jones, E. F., Ray, K. M., Li, W., Chien, A. J., Mukhtar, R. A., Esserman, L. J., … Hylton, N. M. (2019). Initial experience of dedicated breast PET imaging of ER+ breast cancers using [F-18]fluoroestradiol. NPJ breast cancer, 5, 12. doi.org/10.1038/s41523-019-0107-9.
15. Chagpar, A. B., Middleton, L. P., Sahin, A. A., Dempsey, P., Buzdar, A. U., Mirza, A. N., … Singletary, S. E. (2006). Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Annals of surgery, 243(2), 257–264. doi.org/10.1097/01.sla.0000197714.14318.6f.
16. Vedantham, Sm, O’Connell, A. M., Shi, L., Karellas, A., Huston, A. J., & Skinner, K. A. (2014). Dedicated Breast CT: Feasibility for Monitoring Neoadjuvant Chemotherapy Treatment. J. Clin. Imaging Sci., 4, 64. doi: 10.4103/2156-7514.145867.
17. Peintinger, F., Kuerer, H. M., Anderson, K., Boughey, J. C., Meric-Bernstam, F., Singletary, S. E., … Symmans, W. F. (2006). Accuracy of the combination of mammography and sonography in predicting tumor response in breast cancer patients after neoadjuvant chemotherapy. Annals of surgical oncology, 13(11), 1443–1449. doi.org/10.1245/s10434-006-9086-9.
18. Spring, L. M., Fell, G., Arfe, A., Sharma, C., Greenup, R., Reynolds, K. L., & Bardia A. (2020). Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res., 26(12), 2838–2848. doi: 10.1158/1078-0432.CCR-19-3492.
19. Asaoka, M., Narui, K., Suganuma, N., Chishima T., Yamada A., Sugae, S., … Ishikawa T. (2019). Clinical and pathological predictors of recurrence in breast cancer patients achieving pathological complete response to neoadjuvant chemotherapy. Eur. J. Surg. Oncol., 45(12), 2289–2294. doi: 10.1016/j.ejso.2019.08.001.
20. Giffoni de Mello Morais Mata, D., Chehade, R., Hannouf, M. B., Raphael, J., Blanchette, P., Al-Humiqani, A., & Ray, M. (2023). Appraisal of Systemic Treatment Strategies in Early HER2-Positive Breast Cancer-A Literature Review. Cancers (Basel),15(17), 4336. doi: 10.3390/cancers15174336.
21. Avierin, D., & Zavizion, V. (2023). Metformin as an adjuvant option for systemic breast cancer treatment. Med. perspekt., 28(4), 87–96. Available from: journals.uran.ua/index.php/2307-0404/article/view/294154.
22. Gouel, P., Callonnec, F., Levêque, É., Valet, C., Blôt, A., Cuvelier, C., … Viard B. (2023). Evaluation of the capability and reproducibility of RECIST 1.1. measurements by technologists in breast cancer follow-up: a pilot study. Sci. Rep. 13, 9148. doi.org/10.1038/s41598-023-36315-w.
23. Agostinetto, E., Gligorov, J., & Piccart, M. (2022). Systemic therapy for early-stage breast cancer: learning from the past to build the future. Nature reviews. Clinical oncology, 19(12), 763–774. doi.org/10.1038/s41571-022-00687-1.
24. Di Cosimo, S., La Verde, N., Moretti, A., Cazzaniga, M. E., Generali, D., Bianchi, G. V., … de Braud, F. (2019). Neoadjuvant eribulin mesylate following anthracycline and taxane in triple negative breast cancer: Results from the HOPE study. PLoS One, 14(8), e0220644. doi: 10.1371/journal.pone.0220644.
25. Greenwell, K., Hussain, L., Lee, D., Bramlage, M., Bills, G., Mehta, A., … Wexelman, B. (2020). Complete pathologic response rate to neoadjuvant chemotherapy increases with increasing HER2/CEP17 ratio in HER2 overexpressing breast cancer: analysis of the National Cancer Database (NCDB). Breast cancer research and treatment, 181(2), 249–254. doi.org/10.1007/s10549-020-05599-1.
Correspondence:
Dmytro Avierin
9 Volodymyr Vernadsky str., Dnipro, 49044
Dnipro State Medical University
E-mail: avierin@ukr.net
Адреса для листування:
Аверін Дмитро Ігорович
49044, Дніпро, вул. В. Вернадського, 9
Дніпровський державний медичний університет
E-mail: avierin@ukr.net














Leave a comment