Neoadjuvant chemotherapy in resectable locoregionally advanced squamous cell carcinoma of the oral cavity and oropharynx
Burtyn O.V., Kravets O.V., Hilka O.O., Smolanka I.I. , Cherniienko V.V.
Summary. One of the crucial goals of treating oral squamous cell carcinoma (OSCC) and oropharyngeal squamous cell carcinoma (OPSCC) is organ preservation in order to maintain vital functions such as speaking, breathing, swallowing, and chewing. Aim. To investigate the effectiveness of neoadjuvant chemotherapy (CT) and the possibility of organ-preserving treatment in patients with resectable locoregionally advanced squamous cell carcinoma of the oral cavity and oropharynx. Materials and methods. The outcomes of 61 patients with locoregionally advanced squamous cell carcinoma of the oral cavity and oropharynx were analysed. Of them, 42 patients (69%) were diagnosed with stages III-IVa OSCC and 19 (31%) with stages II-IVa OPSCC. Results. Evaluation of the tumour response after neoadjuvant CT in patients with OSCC and OPSCC was performed according to RECIST 1.1 criteria. The findings showed a complete response in 10 (24%) and 7 (37%) patients, a partial response in 12 (28%) and 9 (47%) patients, and stable disease in 13 (24%) and 3 (16%) patients, respectively. Disease progression was seen in 10 (24%) OSCC patients, but none of the OPSCC patients. Conclusions. Following neoadjuvant CT, organ-preserving therapy was administered to 16 (38%) patients with OSCC and 16 (84%) patients with OPSCC. In order to select patients for organ-preserving treatment, further research is needed to identify specific regulatory biomarkers associated with the sensitivity of OSCC and OPSCC to neoadjuvant CT.
Received 31.10.2023
Accepted for publication 10.11.2023
DOI: 10.32471/clinicaloncology.2663-466X.51-3.31122
Over the past decades, there have been substantial changes in approaches to the combined treatment of squamous cell carcinoma of most head and neck sites. Non-surgical organ-preserving approaches involving the use of concurrent chemoradiotherapy (CRT) and neoadjuvant CT followed by radiotherapy (RT) have demonstrated efficacy and are used to treat patients with resectable locally advanced squamous cell carcinoma of the larynx, laryngopharynx and oropharynx [1–3].
Surgery followed by RT/CRT remains the standard of care for patients with resectable locoregionally advanced oral squamous cell carcinoma [4]. The results of two randomised trials suggest that the use of neoadjuvant CT in stage III and IV patients with resectable OSCC did not improve 5-year disease-free survival or overall survival rates compared with surgery and adjuvant RT/CRT. However, researchers observed a decrease in the incidence of mandibular resection after neoadjuvant CT [5–7].
Currently, there is insufficient evidence to support the routine use of neoadjuvant CT before surgery in patients with resectable locoregionally advanced squamous cell carcinoma of the head and neck [8]. However, the paradigm of neoadjuvant treatment for patients with resectable locoregionally advanced squamous cell carcinoma of the oral cavity and oropharynx remains attractive, especially when considering the potential for selecting patients for organ-preserving treatment.
The study aimed to investigate the efficacy of neoadjuvant CT and the potential for organ-preserving treatment in resectable locoregionally advanced OSCC and OPSCC.
MATERIALS AND METHODS
A prospective study analysed the treatment outcomes of 61 patients diagnosed with locoregionally advanced OSCC and OPSCC, who received treatment at the Head and Neck Tumours Research and Clinical Department of the Oncosurgery Clinic of the National Cancer Institute between 2018 and 2020. Forty-two patients (69%) were diagnosed with OSCC in stages III–IVa and 19 patients (31%) were found to have OPSCC in stages II–IVa. The eighth edition of the TNM classification (8th ed., 2017) was utilised to determine the disease stage.
The study was conducted in accordance with the principles of the Declaration of Helsinki and the study protocol was approved by the Ethics Committee of the National Cancer Institute.
The study included patients with resectable OSCC in stages III–IV and OPSCC in stages II–IV who had not undergone prior surgery, RT or chemoradiation treatment.
Exclusion criteria were as follows: non-squamous cell carcinoma of the oral cavity; stage I or II malignancy (except for stage II human papillomavirus-positive OPSCC); a history of other malignancies; the presence of distant metastases; and a patient’s condition deemed a contraindication to neoadjuvant CT.
Among the patients enrolled in the study, there were 44 men (72%) and 17 women (28%). The mean age of patients was 57.2±8.5 years (ranging from 35 to 75 years). Tumour differentiation grade G1 was established in 15 patients (24.6%), grade 2 (G2) was found in 38 patients (62.3%), and 8 patients (13.1%) had grade 3 (G3). Based on the extent of the OSCC process: Stage III (T1-2N1M0, T3N0-1M0) was diagnosed in 9 patients (23%) and Stage IVa (T1N2M0, T2N2M0, T3N2M0, T4aN0-2M0, T1-4N3M0) — in 30 patients (77%). According to the extent of HPV-positive OPSCC: four patients (57%) had Stage II (T1-2N2M0, T3N0-2M0) and three patients (43%) had Stage III (T1-3N3M0, T4N1-3M0). According to the extent of HPV-negative OPSCC: Stage III (T3N0-1M0, T1N1M0, T2N1M0) was diagnosed in 6 (50%) and Stage IVa (T1N1M0, T1-4aN3M0, T2N2M0, T3N2M0, T4aN0-1M0) — in 6 patients (50%).
In the study, HPV-positive OPSCC was diagnosed in 7 patients (37%), and HPV-negative OPSCC was found in 12 patients (63%). The HPV-status of the tumour was determined by immunohistochemistry.
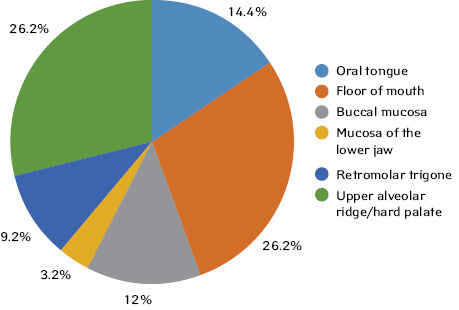
The distribution of patients by site of OSCC was as follows:
- oral tongue — 9 (14.4%) patients;
- floor of mouth — 11 patients (26.2%);
- buccal mucosa — 5 patients (12%);
- mucosa of the lower jaw — 4 patients (9.5%);
- retromolar trigone — 2 patients (4.7%);
- upper alveolar ridge/hard palate — 11 patients (26.2%) (Fig. 1).
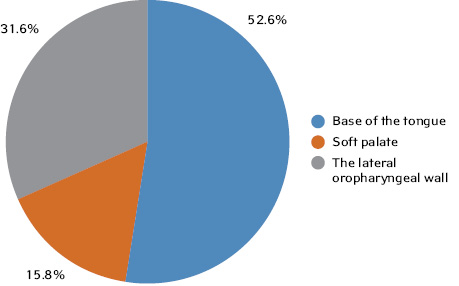
The distribution of patients by OPSCC location was as follows:
- base of the tongue — 10 patients (52.6%);
- soft palate — 3 patients (15.8%);
- the lateral wall of the oropharynx — 6 patients (31.6%) (Fig. 2).
In our study, all patients received three cycles of neoadjuvant CT according to the TPF regimen (intravenous cisplatin 100 mg/m2 on day 1; intravenous 5-fluorouracil 1000 mg/m2 per day as a continuous infusion on days 1 to 4). The interval between neoadjuvant CT cycles was 3 weeks.
Tumour response was assessed according to RECIST 1.1 (Response Evaluation Criteria in Solid Tumours, 2009) three weeks after completion of the third cycle of neoadjuvant CT.
The degree of haematological toxicity was determined in line with the WHO using the Common Toxicity Criteria of the National Cancer Institute.
Patient characteristics are shown in Table 1.
| Characteristic | n (%) | |||
| Age, years | 57.2±8.5 | |||
| Sex | Female | 17 (28) | ||
| Male | 44 (72) | |||
| Site | Oral cavity | upper alveolar ridge/hard palate | 11 (18) | |
| floor of mouth | 11 (18) | |||
| mucosa of the lower jaw | 4 (6.5) | |||
| retromolal trigone | 2 (3.2) | |||
| buccal mucosa | 5 (8.2) | |||
| oral tongue | 9 (14.7) | |||
| Oropharynx | base of the tongue | 10 (16.4) | ||
| soft palate | 3 (5) | |||
| the lateral oropharyngeal wall | 6 (10) | |||
| T | Oral cavity | T2 | 2 (3.2) | |
| T3 | 16 (26.2) | |||
| T4a | 24 (39.3) | |||
| Oropharynx | HPV+ | T2 | 4 (6.5) | |
| T3 | 2 (3.2) | |||
| T4 | 1 (1.6) | |||
| HPV- | T2 | 3 (5) | ||
| T3 | 3 (5) | |||
| T4a | 6 (9.8) | |||
| N | Oral cavity | N0 | 14 (23) | |
| N1 | 19 (31.1) | |||
| N2a-c | 8 (13.1) | |||
| N3a-b | 1 (1.6) | |||
| Oropharynx | HPV+ | N0 | 1 (1.6) | |
| N1 | 3 (5) | |||
| N2 | 3 (5) | |||
| N3 | 0 | |||
| HPV- | N0 | 2 (3.2) | ||
| N1 | 6 (9.8) | |||
| N2a-c | 3 (5) | |||
| N3a-b | 1 (1.6) | |||
| Stage | Oral cavity | ІІІ | 12 (19.6) | |
| VIа | 30 (49.1) | |||
| Oropharynx | HPV+ | ІІ | 4 (6.5) | |
| III | 3 (5) | |||
| HPV- | ІІІ | 6 (9.8) | ||
| VIа | 6 (9.8) | |||
RESULTS
Sixty-one patients met the inclusion criteria. Of these, 58 patients (93.5%) received three cycles of neoadjuvant CT according to the TPF regimen and three patients (6.5%) underwent two courses; three patients (5%) did not receive the third course of neoadjuvant CT due to grade III haematological toxicity.
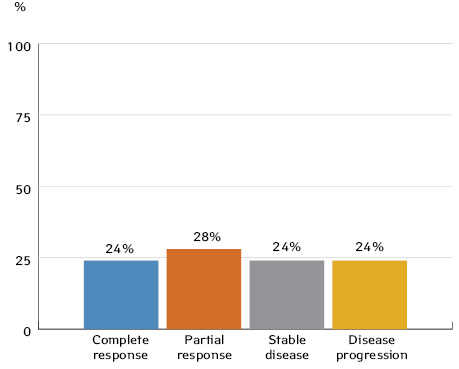
Tumour response was evaluated 3 weeks after completion of the third cycle of neoadjuvant CT and in three patients (6.5%) after completion of the second cycle of neoadjuvant CT. According to RECIST 1.1 criteria, a complete tumour response following neoadjuvant CT was seen in 17 patients (28%) with OSCC or OPSCC, 21 patients (34.4%) had a partial response; 13 patients (21.3%) had stable disease, and 10 patients (16.3%) had disease progression.
Figures 3 and 4 show tumour response following neoadjuvant CT in patients with OSCC or OPSCC.
After neoadjuvant CT, the patients were restaged as follows: 19 patients (45%) with OSCC were downstaged, four patients (10%) were upstaged, and 19 patients (45%) had no change in tumour stage. Among patients with OPSCC, 16 patients (84%) were downstaged, no patients were upstaged, and three patients (16%) had no change in tumour stage. Tables 2 and 3 demonstrate the characteristics of cTN and ycTN, respectively.
| No | cTN | ycTN | Restaging |
| 1 | Т3N0 | T0N0 | ↓ |
| 2 | Т3N0 | T0N0 | ↓ |
| 3 | Т2N1 | T1N0 | ↓ |
| 4 | Т3N1 | T2N0 | ↓ |
| 5 | Т4аN1 | T0N0 | ↓ |
| 6 | Т3N0 | T0N0 | ↓ |
| 7 | Т4аN1 | Т4aN1 | − |
| 8 | Т4аN1 | Т4aN1 | − |
| 9 | Т4аN1 | Т4аN1 | − |
| 10 | Т4аN0 | Т4аN1 | − |
| 11 | Т4аN0 | Т4аN0 | − |
| 12 | Т4аN0 | Т4аN0 | − |
| 13 | Т4аN0 | Т4аN0 | − |
| 14 | Т4аN2 | Т4аN2 | − |
| 15 | Т4аN1 | Т4аN1 | − |
| 16 | Т4аN3а | Т4аN3а | − |
| 17 | Т4аN1 | Т4аN1 | − |
| 18 | Т4аN0 | Т4аN0 | − |
| 19 | Т4аN1 | Т4аN1 | − |
| 20 | Т4aN2 | Т4aN2 | − |
| 21 | Т4аN2 | Т4bN2 | ↑ |
| 22 | Т4аN1 | Т4bN1 | ↑ |
| 23 | Т3N0 | T0N0 | ↓ |
| 24 | Т3N0 | T0N0 | ↓ |
| 25 | Т4аN1 | T0N0 | ↓ |
| 26 | Т4аN1 | T0N0 | ↓ |
| 27 | Т4аN2 | T0N0 | ↓ |
| 28 | Т3N1 | T2N0 | ↓ |
| 29 | Т3N0 | T1N0 | ↓ |
| 30 | Т3N1 | T1N0 | ↓ |
| 31 | Т3N2 | T2N1 | ↓ |
| 32 | Т3N0 | T2N0 | ↓ |
| 33 | Т3N1 | T2N0 | ↓ |
| 34 | Т3N2с | T2N1 | ↓ |
| 35 | Т3N1 | T3N1 | − |
| 36 | Т2N2 | T4aN2 | − |
| 37 | Т3N2 | T4aN2 | ↑ |
| 38 | Т3N1 | T4aN1 | ↑ |
| 39 | Т4аN1 | T4aN0 | − |
| 40 | Т3N0 | T0N0 | ↓ |
| 41 | Т3N0 | T0N0 | ↓ |
| 42 | Т2N1 | T1N0 | ↓ |
Notes. ↓— downstaged disease, ↑ — upstaged disease, — — no change in stage.
| No | cTN | ycTN | Restaging |
| 1 | Т2N1 | T0N0 | ↓ |
| 2 | Т3N1 | T2N0 | ↓ |
| 3 | Т3N0 | T1N0 | ↓ |
| 4 | Т3N0 | T0N0 | ↓ |
| 5 | Т4аN2 | T0N0 | ↓ |
| 6 | Т2N1 | T1N0 | ↓ |
| 7 | Т3N0 | Т2N0 | ↓ |
| 8 | Т2N1 | T1N0 | ↓ |
| 9 | Т2N1 | T1N0 | ↓ |
| 10 | Т2N2 | T0N0 | ↓ |
| 11 | Т4аN2 | T2N1 | ↓ |
| 12 | Т2N2 | T0N0 | ↓ |
| 13 | Т2N3 | T0N0 | ↓ |
| 14 | Т3N1 | T0N0 | ↓ |
| 15 | Т4аN1 | Т4аN1 | − |
| 16 | Т4аN2 | Т4аN1 | − |
| 17 | Т4аN1 | Т4аN1 | − |
| 18 | Т4аN1 | Т3N1 | ↓ |
| 19 | Т4аN2 | Т2N0 | ↓ |
Notes. ↓ — downstaged disease, ↑ — upstaged disease, — — no change in stage.
Ten patients (24%) with OSCC underwent non-surgical organ-preserving treatment (neoadjuvant CT + RT). They had a complete tumour response after neoadjuvant CT. A residual tumour after neoadjuvant CT and RT was detected in two patients (4.7%). After two to three months following CRT, these patients underwent organ-preserving transoral CO2-laser microsurgery (Fig. 5). These patients avoided extended surgical interventions that would have been performed if the primary surgical approach had been used, such as resection of the maxilla with orbital exenteration in one case (2.4%), mandibular resection in seven cases (16.7%), and subtotal resection of the tongue in two cases (4.8%).
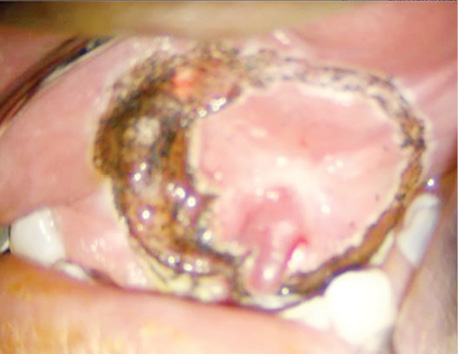
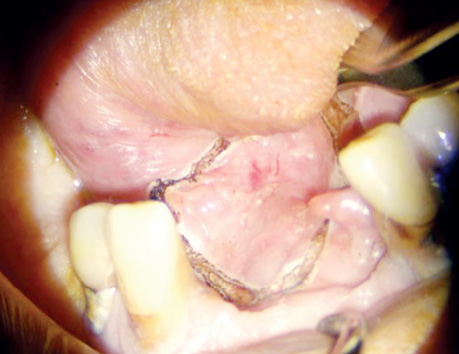
Organ-preserving surgeries after neoadjuvant CT were performed in six patients (14%). They demonstrated a partial tumour response. Among them, one patient (2.4%) avoided orbital exenteration, three patients (7.1%) avoided mandibular resection, one patient (2.4%) avoided maxillary resection, and one patient (2.4%) avoided subtotal tongue resection. One patient (2.4%) underwent surgical treatment alone. Three patients (7.1%) received adjuvant RT, and one patient (2.4%) underwent concurrent CRT.
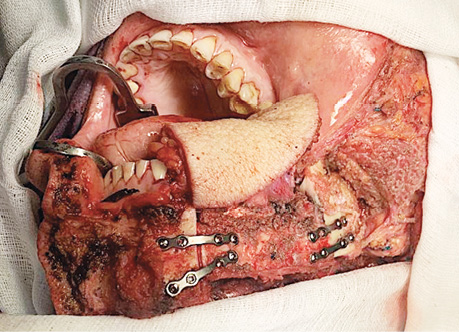
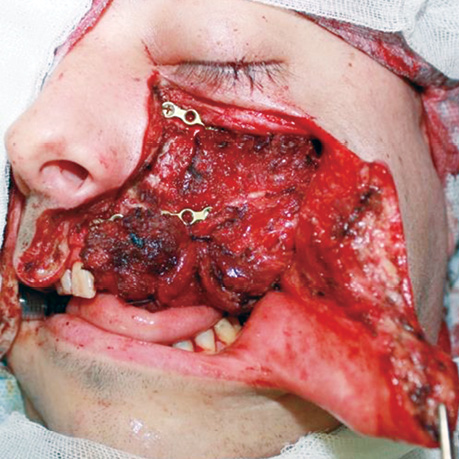
Six patients (14%), who had a partial response, and 10 patients (24%), who had stable disease following neoadjuvant CT, underwent extended combined surgeries (Figs. 6, 7). Thirteen patients (31%) received adjuvant RT, and three patients (7%) had concurrent CRT. Adjuvant CRT was administered to patients with positive resection margins and/or extranodal extension.
Out of 10 patients (24%) who had disease progression after neoadjuvant CT, 4 patients (9.5%) underwent extended combined surgeries, two (4.8%) received palliative RT, and four (9.5%) had palliative CT.
In total, 16 patients (38%) with OSCC underwent organ-preserving treatment after neoadjuvant CT.
Non-surgical organ-preserving treatment (neoadjuvant CT + RT) was administered to 16 patients (84%) with OPSCC, who demonstrated a complete or partial tumour response after neoadjuvant CT. A residual tumour after neoadjuvant CT and RT was detected in four patients (21%). These patients underwent organ-preserving transoral CO2-laser microsurgery after 2 to 4 months following CRT (Fig. 8).
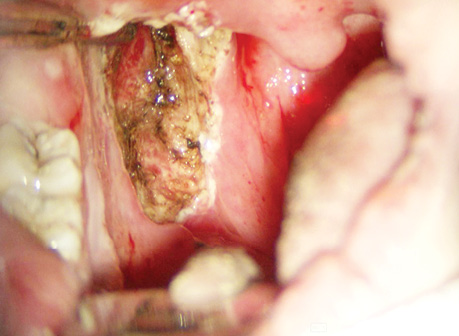
Out of three patients (16%) with stable disease after neoadjuvant RT, two patients (10.5%) had extended surgeries and one patient (5.5%) received RT. After surgery, two patients (10.5%) underwent adjuvant RT and no patients received concurrent CRT.
In total, 16 patients (84%) with OPSCC received organ-preserving treatment after neoadjuvant CT.
DISCUSSION
Neoadjuvant CT offers some theoretical advantages, in particular, optimal distribution of the drug in the tumour under conditions of unimpaired blood supply, an early effect on micrometastases and better drug tolerability. Furthermore, neoadjuvant CT makes it possible to evaluate the tumour response and select patients for organ-preserving treatment. Various neoadjuvant CT regimens have been used for years, but according to the MACH-NC meta-analysis, the cisplatin + 5-fluorouracil regimen was found to be the most effective (the docetaxel/paclitaxel + cisplatin + 5-fluorouracil regimen trial was not included in the meta-analysis) [9]. Subsequent studies demonstrated the benefit of the docetaxel/paclitaxel + cisplatin + 5-fluorouracil regimen compared with cisplatin + 5-fluorouracil in the treatment of stage III–IV squamous cell carcinoma of the head and neck [10].
Two randomised phase III studies investigated the role of neoadjuvant CT in the treatment of patients with resectable oral cancer. In the Italian study, 195 patients with resectable OSCC in stages II–IV were randomised into two groups. Patients in the first group received three cycles of neoadjuvant CT with the use of the cisplatin + 5-fluorouracil regimen followed by surgery. Patients in the second group underwent surgical treatment. In both groups, patients with unfavourable prognostic factors received CT after surgery. Despite the fact that 27% of patients achieved a complete clinical response after three cycles of neoadjuvant CT, no significant differences were found in 5-year overall survival, locoregional recurrence rate or distant metastases. However, L. Licitra et al. demonstrated a decrease in the frequency of mandibular resection after neoadjuvant CT. An analysis of the 10-year results of the study confirmed these findings [11].
L. Zhong et al. studied 256 patients with resectable stage III–IV OSCC who were also randomised into two groups. Patients in the first group received two cycles of neoadjuvant CT with the docetaxel + cisplatin + 5-fluorouracil regimen, followed by surgery and postoperative RT. Patients in the second group underwent surgical treatment and postoperative RT. In contrast to the previous study, a complete clinical response was observed in 13.4% of patients treated with neoadjuvant CT. Similar to the Italian study, no significant differences were found in overall survival, disease-free survival or locoregional control. The reduction in the incidence of distant metastases in patients treated with neoadjuvant CT was not statistically significant [6].
In our study, 24% of patients with locoregionally advanced OSCC achieved a complete clinical response following 3 cycles of neoadjuvant CT (compared to 27% in the study by L. Licitra et al. and 13.4% in the study by L. Zhong et al.). Besides, similar to L. Licitra et al., we observed a decrease in the frequency of mandibular resection, maxillary resection and subtotal tongue resection.
Q. Zhang et al. showed that after 3 cycles of neoadjuvant CT in patients with locoregionally advanced OSCC, 41.7% of patients achieved a complete clinical response and 49.1% of patients had a partial response [12].
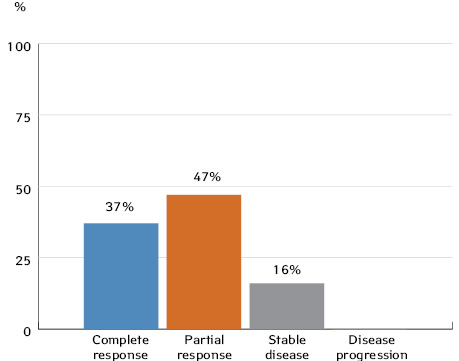
We obtained similar results: after 3 cycles of neoadjuvant CT, 37% of patients with locoregionally advanced OPSCC had a complete clinical response and 47% showed a partial response.
Along with primary surgery, neoadjuvant CT is presently the treatment of choice for patients with resectable locoregionally advanced OPSCC, but it is not advisable for locoregionally advanced OSCC. However, the use of neoadjuvant CT allows the evaluation of tumour responses to therapy and the selection of patients for organ-preserving treatment. It is noteworthy that the lack of methods for predicting the effectiveness of neoadjuvant CT makes timely radical surgical treatment a challenge for patients with locoregionally advanced OSCC who have stable or progressing disease and account for almost 50%, according to the above studies.
An in-depth study of the molecular biological factors implicated in the development of radiochemical resistance in the tumour and the factors indicating the effectiveness of certain groups of chemotherapeutic drugs has provided the basis for predicting of the disease course and tumour response to treatment. The study of epigenetic abnormalities in the occurrence and progression of malignancies, including OSCC and OPSCC has been one of the most relevant areas of fundamental oncology over the past few years [13].
Recent studies have shown that the initiation and progression of malignant tumours are characterised by changes in the ratio of epigenetic biomarkers, namely microRNAs, as they are the main regulators of genes involved in carcinogenesis [14]. The study of microRNA expression patterns in tumour cells and biological fluids (blood, saliva, urine, etc.) is informative for early differential diagnosis of malignancies, verification of the histological origin of tumours, determination of the tumour grade and sensitivity to drug therapy [15]. Tumour-associated microRNAs have already been shown to have high diagnostic sensitivity and specificity for a variety of malignancies and can be used as non-invasive prognostic and predictive markers in medical practice based on the results of clinical observations and available experimental data [16–17]. However, no studies have been conducted to identify the microRNAs associated with the sensitivity of OSCC and OPSCC to neoadjuvant CT.
Overall, the identification of specific regulatory microRNAs associated with tumour sensitivity to neoadjuvant CT will provide the basis for the development of innovative strategies for personalised treatment.
CONCLUSIONS
1. In cases of resectable locoregionally advanced OSCC and OPSCC, neoadjuvant CT can provide a partial response in 28% and 47% of patients, respectively, and a full response in 24% and 37% of patients.
2. Of the patients with locoregionally advanced OSCC and OPSCC, 38% and 84%, respectively, received organ-preserving therapy.
3. In order to select patients for organ-preserving treatment, further research is needed to identify specific regulatory biomarkers associated with the sensitivity of OSCC and OPSCC to neoadjuvant CT.
REFERENCES
1. Gillison, M. L., Trotti, A. M., Harris, J., Eisbruch, A., Harari, P. M., Adelstein, D. J., & Quynh, T. L. (2019). Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet, 393(10166), 40–50. doi: 10.1016/S0140-6736(18)32779-X.
2. Geoffrois, L., Martin, L., De Raucourt, D., Shan Sun, X., Tao, Y., … Bourhis, J. (2018). Induction Chemotheapy followed by Cetuximab radiotherapy is not superior to concurrent Chemoradiotherapy for head and neck carcinomas: results of the GORTEC 2007–02 phase III randomized trial. Journal of Clinical Oncology, 36(31), 3077–3083. doi: 10.1200/JCO.2017.76.2591.
3. Kiong, K. L., de Souza, N. N., Sultana, R., & Iyer, N. G. (2018). Meta-analysis of induction chemotherapy as a selection marker for chemoradiation in the head and neck. Laryngoscope, 128(7), 1594–601. doi: 10.1002/lary.27011.
4. D’Cruz, A. K., Vaish, R., & Dhar, H. (2018). Oral cancers: Current status. Oral Oncology, 87, 64–69. doi: 10.1016/j.oraloncology.2018.10.013.
5. Bossi, P., Lo Vullo, S., Guzzo, M., Mariani, L., Granata, R., Orlandi, E., … Licitra, L. (2018). Preoperative chemotherapy in advanced resectable OCSCC: long-term results of a randomized phase III trial. Annals of Oncology, 25(2), 462–466. doi: 10.1093/annonc/mdt555.
6. Zhong, L. P., Zhang, C. P., Ren, G. X., Guo W., William, N., William, Jr., … Zhang, Z. Y. (2015). Long-term results of a randomized phase III trial of TPF induction chemotherapy followed by surgery and radiation in locally advanced oral squamous cell carcinoma. Oncotarget, 6(21), 18707–18714. doi: 10.18632/oncotarget.4531.
7. Marta, G. N., Riera, R., Bossi, P., Zhong, L. P., Licitra, L., Macedo, C. R., … Kowalski, L. P. (2015). Induction chemotherapy prior to surgery with or without postoperative radiotherapy for oral cavity cancer patients: Systematic review and meta-analysis. European Journal of Cancer, 51(17), 2596–2603. doi: 10.1016/j.ejca.2015.08.007.
8. Stafford, M., & Kaczmar, J. (2020). The neoadjuvant paradigm reinvigorated: a review of pre-surgical immunotherapy in HNSCC. Cancers of the Head & Neck, 5, 4. doi: 10.1186/s41199-020-00052-8.
9. Blanchard, P., Baujat, B., Holostenco, V., Bourredjem, A., Baey, C., Bourhis, J., & Pignon, J. P. (2011). Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiotherapy&Oncology, 100(1), 33–40. doi: 10.1016/j.radonc.2011.05.036.
10. Lorch, J. N., Goloubeva, O., Haddad, R. J., Cullen, K., Sarlis, N., Tishler, R., … Posner, M. P. (2011). Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX324 randomised phase 3 trial. The Lancet Oncology, 12(2), 153–159. doi: 10.1016/S1470-2045(10)70279-5.
11. Bossi, P., Vullo, S. L., Guzzo, M., Mariani, L., Granata, R., Orlandi, E., … Licitra, L. (2014). Preoperative chemotherapy in advanced resectable OCSCC: long-term results of a randomized phase III trial. Annals of Oncology, 25(2), 462–466. doi: 10.1093/annonc/mdt555.
12. Zhang, O., Zhou, X., Tingting, X., Shen, C., Ying, H., Xiayun, H., & Xueguan, L. (2022). The impact of induction chemotherapy response to survival outcomes in oropharyngeal cancer. Journal of Clinical Oncology, 40(16), 6040. doi: 10.1200/JCO.2022.40.16_suppl.6040.
13. Falzone, L., Lupo, G., La Rosa, G. R. M., Crimi, S., Anfuso, C. D., Salemi, R., … Candido, S. (2019). Identification of Novel MicroRNAs and Their Diagnostic Significance in Oral Cancer. Cancers (Basel), 11(5), 610. doi: 10.3390/cancers11050610.
14. Zeljic, K., Jovanovic, I., Jovanovic, J., Magic, Z., Stankovic, A., & Supic, G. (2018). MicroRNA meta-signature of oral cancer: evidence from a meta-analysis. Upsala Journal of Medical Science, 123(1), 43–49. doi: 10.1080/03009734.2018.1439551.
15. Schneider, A., Berta, V., Lopez, V. B., Suchorska, Y. N., Barczak, W., Sobecka, A., & Golusinski, P. (2018). Tissue and serum microRNA profile of oral squamous cell carcinoma patients. Scientific Reports, 8(1), 1–8. doi: 10.1038/s41598-017-18945-z.
16. Lekka, E., & Hall, J. (2018). Noncoding RNAs in disease. FEBS Letters, 592(17), 2884–2900. doi: 10.1002/1873-3468.13182.
17. Lukianova, N., Borikun, T., Bazas, V., Yalovenko, T., Zadvornyi, T., Malyshok, N., & Rossilna, O. (2019). Circulating microRNAs: prospects for use in early diagnosis and monitoring of tumour progression. Oncology, 21(3), 181–191. doi: 10.32471/oncology.2663-7928.t-21-3-2019-g.8001.
Correspondence:
Olga Burtyn
33/43 Yulia Zdanovska str., Kyiv, 03022
Nonprofit Organization National Cancer Institute
E-mail: olyabyrtun@gmail.com
Адреса для листування:
Буртин Ольга
03022, Київ, вул. Здановської Юлії, 33/43
Державне некомерційне підприємство«Національний інститут раку»
E-mail: olyabyrtun@gmail.com














Leave a comment